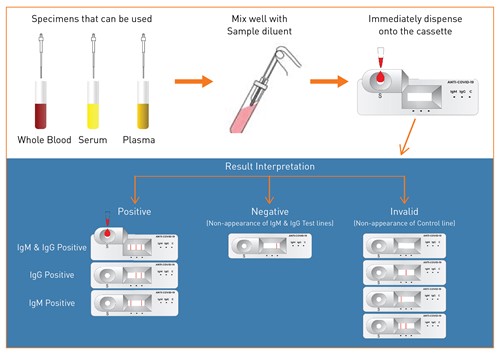
The First Reliable and Rapid Antibody based Test
- -Qualitative detection of IgM and IgG antibodies against COVID-19 in human serum, plasma, and whole blood
- -Based on Lateral flow immunochromatographic principle
- -Results in less than 10 minutes
- -High Sensitivity (97.4%) & Specificity (98%)
Technical specifications
- -Simultaneous surveillance and detection of both the Covid-19 antibodies (IgM and IgG), aiding the diagnosis of present and past infection
- -Highly Sensitive (97.4%) and robust test enabling the detection of infection even at low levels of antibody concentration
- -Highly Specific (98%) and no cross reactivity observed with several bacterial, viral and parasitic microorganisms
- -No inhibition or false positive results when tested with several potential inhibitory substances
- -Straightforward result interpretation
A solid-phase enzyme immunoassay for the qualitative detection of IgG / IgA / IgM antibodies to Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human serum or plasma. The positive result is an aid for the diagnosis of acute or early SARS-CoV-2 infection in human.
- -Qualitative detection of COVID (SARS-Cov-2) IgG/IgA/IgM EIA Antibody
- -Principle based on an indirect solid-phase enzyme immunoassay
- -Sample Type – Serum / Plasma
- -Available in convenient pack size of 96 Tests
- -Longer Shelf Life: 12 months at 2° C - 8° C
Technical Specifications
- -The Kit can be used for diagnostic as well as serological surveillance purposes
- -Compatible with major semi & fully automated ELISA analysers
- -The Kit is based on an indirect solid-phase enzyme immunoassay with horseradish peroxidase as a marker enzyme.
- -Colour coded reagents for ease of identification
- -Negative & Positive Control included in kits
Kit Contents
- -COVID-19 Antigen Coated Microplate
- -Sample Diluent
- -Negative Control
- -Cut-Off Control
- -Positive Control
- -Conjugate
- -TMB / Substrate Solution
- -Stopping Solution
- -Washing Solution
| P/N | Product Description | Pack Size |
|
LS-6111700 |
Covid IgG EIA |
96 tests |
|
LS-6111710 |
Covid IgA EIA |
96 tests |
|
LS-6111720 |
Covid IgM EIA |
96 tests |
-Simultaneous detection of 3 specific COVID-19 genes ORF1ab, N & E besides internal control.
- -Low virus load detection: < 5 copies/ µl
- -Extracted RNA samples from entire respiratory tract can be used
- -Compatible with multiple RT-PCR Instruments
Technical specifications
- -Highly Specific: No detectable cross reactivity with most human Coronavirus strains, respiratory pathogens, etc
- -Utmost Sensitivity:
- -Wider range of Covid-19 strains covered to detect most mutations (mutation variants)
- -Tested and certified raw material with an optimal assay design to achieve low detection levels
- -Timesaving Approach: Processing time is approximately 1 hour
- -Certified Quality: CE IVD marked reliable quality from Northern Europe
- -Compatible RT-PCR Instruments: Most common RT-PCR instruments can be used such as ABI Prism 7500/7900 from Life Technologies; CFX96 from Bio-Rad; Rotor-Gene 6000 from Qiagen
- -RNA Isolation: RT-PCR is compatible with several common RNA isolation kits
- -Specific RNA isolation kits can also be ordered from Labsystems Diagnostics
- -Shelf life: 12 months
Ready to use reagent:
- -Covid-19 Reaction mix
- -Covid-19 Primer Probe Mix
- -Covid-19 Positive control
- -Covid-19 Negative control
Availability
Kit sizes of 50 and 100 tests with a great value proposition


